Alejandro K. Samhan-Arias1,*, Carmen López-Sánchez2,*, Dorinda Marques-da-Silva1, Ricardo Lagoa1,2,3, Virginio Garcia-Lopez2,4, Virginio García-Martínez2 and Carlos Gutierrez-Merino1,#
1Dept. Biochemistry and Molecular Biology, Faculty of Sciences, University of Extremadura, 06006-Badajoz, Spain.
2Dept. Human Anatomy and Embryology, Faculty of Medicine, University of Extremadura, 06006-Badajoz, Spain.
3ESTG- Polytechnic Institute of Leiria, Leiria, Portugal
4FARMADIEX 06008 Badajoz, Spain
Cytochrome b5 reductase (Cb5R) and cytochrome b5 (Cb5) are coupled redox systems with a high potential as biomarkers of health and disease in the brain because they regulate metabolic pathways that are essential to maintain normal neuronal function, like lipid biosynthesis, steroid and xenobiotics metabolism, neuronal bioenergetics and production of reactive oxygen species. Mutations of the Cb5R reported in humans produce recessive congenital methemoglobinemia of type II, a disease with severe clinical neurological dysfunctions. The isoform 3 of Cb5R (Cb5R3) and Cb5 are highly expressed in pyramidal neurons of the primary and secondary motor areas of frontoparietal cerebral cortex, hippocampus, vestibular, reticular and motor nuclei of the cerebellum and brain stem, and also in Purkinje and granule neurons of the cerebellum cortex. These brain areas are highly prone to undergo oxidative stress-induced neurodegeneration and their functional impairment can account for neurological deficits reported in type II congenital methemoglobinemia.
The redox system Cb5/Cb5R acts as an electron carrier coupled to cytosolic NADH consumption in endoplasmic reticulum, mitochondria and plasma membrane of mammalian cells. Cb5 is a pleiotropic co-factor of multiple enzymes and redox chains that play critical roles for normal function of healthy mammalian organisms, and it is largely reduced by the NADH-dependent Cb5R activity (reviewed in Samhan-Arias and Gutierrez-Merino1).
Neurons have an extremely high dependence on lipid metabolism to make new synaptic connections and also for synaptic plasticity and activity. In mammalian cells Cb5 and Cb5R modulate palmitoil-CoA elongation and desaturation and cholesterol synthesis1, and also the dihydroceramide:sphinganine C-4 hydroxylase2. Moreover, reduced Cb5 has been shown to activate sialic acid metabolism3, a metabolite required for the synthesis of gangliosides, a major family of brain lipids. In addition, Cb5 is also a cofactor of NADPH-dependent cytochrome P450 monooxygenation reactions involved in steroid and xenobiotics metabolism4,5. Owing to the relevance of oxidative stress in neurodegenerative processes, it is to be noted that the potentiation by Cb5 of the metabolism through cytochrome P450s decreases the rate of collateral reactions releasing non-productive superoxide anion and its dismutation metabolite hydrogen peroxide4. Taking into account the relevance of endoplasmic reticulum stress in Alzheimer’s disease6,7, it is to be noted that the Cb5R isoform 4 (Cb5R4) - a flavohemeprotein containing Cb5 and Cb5R domains- has been shown to protect against endoplasmic reticulum stress-induced lipotoxicity8.
As an impaired neuronal bioenergetics is a metabolic alteration shared in many neurodegenerative diseases, it is of interest to recall here that the NADH oxidase activity of the Cb5R bound to the external mitochondrial membrane has been proposed to contribute in the maintenance of adequate levels of cellular ATP, via direct reduction of cytochrome c through an electron shuttle with cytochrome c oxidase9. Furthermore, the mitochondrial complex Cb5R/Cb5 contributes to the cobalamin(III) reduction for the biosynthesis of adenosylcobalamin10, and in the presence of Cb5 microsomal cytochrome P450 reductase activates methionine synthase, catalyzing the formation of methylcobalamin and production of methionine from homocysteine and 5-methyltetrahydrofolate11.
In addition, Cb5R has been shown to be a component of the ‘so-called’ redox chain of the plasma membrane in mammalian cells, which plays a major role in the recycling of extracellular ascorbate from ascorbate-free radical12. Because extracellular ascorbate is a major antioxidant defense in the brain, in previous works we have shown the presence of ascorbate-free radical reductase activity in synaptic membranes13 and the association of Cb5R with the plasma membrane in cerebellar granule neurons in culture14-16. We noticed that the soluble form of Cb5 stimulates the Cb5R associated with synaptic plasma membranes15. The presence of the cDNAs encoding for a soluble and for a membrane-bound isoform of Cb5 in isolated neuronal and glial cultures was shown by Yoo17, and confirmed by our group in primary cultures of cerebellar granule neurons from rat tissue18. In previous publications14-16,18,19 we have also pointed out that the Cb5R associated with the neuronal plasma membrane is clustered within caveolin-1-rich lipid rafts sub-microdomains or nanodomains and that it is the isoform 3 of Cb5R (Cb5R3) encoded by the gene CB5R3 or DIA-1.
Mutations of the gene CB5R3 (or DIA-1) encoding for Cb5R that cause deficiency of the NADH-dependent enzyme activity are known to produce recessive congenital methemoglobinemia20. More than 50% of the mutations of the Cb5R reported in humans produce recessive congenital methemoglobinemia of type II, an inherited disease characterized by mild cyanosis, developmental delay, severe neurological impairment and reduced life expectancy20-23. In erythrocytes, methemoglobin is kept by two systems at levels below 1% of total hemoglobin24. The NADH-dependent Cb5R activity accounts for more than 95% of the erythrocyte reducing capacity of methemoglobin, as methemoglobin forms a bimolecular complex with reduced Cb5 that leads to its reduction to hemoglobin25. The second system that can reduce methemoglobin is a NADPH-dependent flavin reductase. This pathway only affords in vivo a minor contribution to the reduction of methemoglobin, because its malfunction do not cause a methemoglobin reduction-deficient phenotype24,25. Clinical neurological dysfunctions of the humans affected by recessive congenital methemoglobinemia of type II include progressive microcephaly, generalized dystonia, movement disorders, failure to thrive, and cortical and subcortical atrophy20-23, including cerebellar atrophy26.
The dysregulation of the neuronal Cb5R3 associated with lipid rafts of the plasma membrane is largely responsible for the early superoxide anion overshot that precedes the activation of caspases in cerebellar granule neurons apoptosis induced by low potassium in the extracellular medium18,27-29, a widely accepted model for the apoptotic death of neurons during in vivo development and in response to stress and neurotoxic insults30. Moreover, the increase of superoxide anion production by Cb5R in this neuronal model of apoptosis correlated with the increase of the expression level of soluble Cb518, which stimulates the activity of Cb5R associated with synaptic membranes15. Indeed, we found that the ratio between the expression of soluble and membrane isoforms of Cb5 changed during the early stages of the apoptosis of these neurons. Noteworthy, the redox properties of brain Cb5 have been reported to be different from those of Cb5 isoforms found in other tissues31. On these grounds, we have proposed that soluble Cb5 produced dysregulation or uncoupling of the plasma membrane-bound Cb5R3, which is largely clustered within plasma membrane lipid rafts in primary cultures of mature cerebellar granule neurons14,15,18.
The concentrations of Cb5 in mammalian cells are not saturating for Cb5R, and, therefore, the early increase of Cb5 levels that we found in low potassium-induced apoptosis of cerebellar granule neurons can fully account for the observed rapid Cb5R stimulation18. This can be seen as a defensive response against cellular stress, because it should help to ensure the maintenance of cellular ATP levels via direct reduction of mitochondrial cytochrome c9. This protective role can be important during aging, which elicits a drop of Cb532. Indeed, it has been shown that calorie restriction, a treatment known to attenuate or to retard the aging process33,34, up-regulates the NADH-dependent reductases activities of the plasma membrane redox system in brain cells, of which Cb5R is a major enzyme component, and attenuate oxidative stress during aging35.
However, Cb5 seems to be one of the major targets for cytochrome c after being released from mitochondria during apoptosis, as they form a complex with a dissociation constant value of approximately 0.1 μM36,37. Thus, release of cytochrome c from mitochondria decreases free Cb5 concentrations at a later stage of apoptosis.
On these grounds, the regionalization of these redox systems in the brain deserves to be analyzed in detail. In this sense, we have recently reported38 that pyramidal neurons of mature rat brain displays a high level of expression of Cb5R3 and Cb5. Noteworthy, large pyramidal neurons of the primary and secondary motor areas of the frontoparietal cerebral cortex and the hippocampus showed an expression level of these proteins much higher than other cells in these brain regions. Our results pointed out that Cb5R3, Cb5 and neurogranin showed a similar staining pattern in cerebral neocortex, in pyramidal soma and dendritic trees. The high level of expression of Cb5R3 and Cb5 in giant pyramidal neurons of the primary motor areas (Betz cells) has a special integrative neurological relevance, as these neurons have very long axons that form synapses with spinal motoneurons and brain stem motor nuclei. In the hippocampus, pyramidal neurons of Ammon´s horn and in particular the soma of CA1 pyramidal neurons were also heavily stained with anti-Cb5R3 and anti-Cb5. The expression level of Cb5R3 is also observable in apical dendrites of the stratum radiatum stained with anti-neurogranin, while the basal dendrites of the stratum oriens appeared only weakly stained with anti-Cb5R3 and with anti-Cb5.
In the cerebellum, we found a high expression of Cb5R3 and Cb5 in the cerebellar cortex, labeling heavily granule neurons and Purkinje cells, and in structures such as the fastigial, interposed and dentate cerebellar nuclei. Purkinje cells, which play a major role in the control of efferent connections of the cerebellar cortex and whose neurodegeneration has been reported to mediate the loss of control functions observed in spinocerebellar and spastic ataxias39,40, express both proteins at levels much higher than other cells present in the cerebellar cortex. Neuronal bodies of the granular layer of the cerebellar cortex also display a dense staining with anti-Cb5R3 and with anti-Cb5, while a dense staining of Bergmann glial cells surrounding the soma of Purkinje neurons was observed with anti-Cb5 but not with anti-Cb5R3, yielding a more diffuse distribution of Cb5 staining between granular and molecular layers of the cerebellar cortex.
Efferent projections of the Purkinje cells are also stained with anti-Cb5R3, anti-Cb5 and anti-calbindin, and reach the vestibular and cerebellar nuclei, where we found that Cb5R3 is also highly expressed38. In particular, we observed heavy staining in motor trigeminal nucleus, hypoglossal nucleus, dorsal motor nucleus of the vagus nerve, spinal nucleus of the trigeminal, ventral cochlear nucleus and facial nerve (root of facial nerve). In the caudal pontine reticular nucleus we found that Cb5R3 was highly expressed in the soma of gigantocellular neurons, which are involved in the control of involuntary movements associated with mastication and grinding of teeth during sleep41. Therefore, the high level of Cb5R3 that we found in vestibular, reticular and motor nuclei located at the brain stem level correlate well with the generalized dystonia and movements disorders noticed in patients suffering type II-recessive congenital methemoglobinemia.
Noteworthy, rat brain regionalization found in our work for Cb5R3 and Cb5 resembles the reported distribution of NADH-dependent dehydrogenases with transplasma membrane oxidoreductase activity42. Consistently, using fluorescence resonance energy transfer imaging we have found that a large part of Cb5R3 in the cerebellum cortex was regionalized in close proximity to the neuronal plasma membrane lipid rafts labeled with cholera toxin B38. This is in good agreement with our previous results showing the presence of a high density network of lipid rafts in the plasma membrane of the soma of cerebellar granule neurons maturated in vitro, to which Cb5R3 is associated15,16,18,19.
The major biochemical and anatomical points focused in this review are summarized in the schematic Figure 1. A major merging conclusion for clinical neurology is that Cb5R3 and Cb5 are highly expressed in neuronal cells and structures of the brain whose functional impairment can account for neurological deficits reported in type II congenital methemoglobinemia, as well as in brain areas highly prone to undergo oxidative stress-induced neurodegeneration.
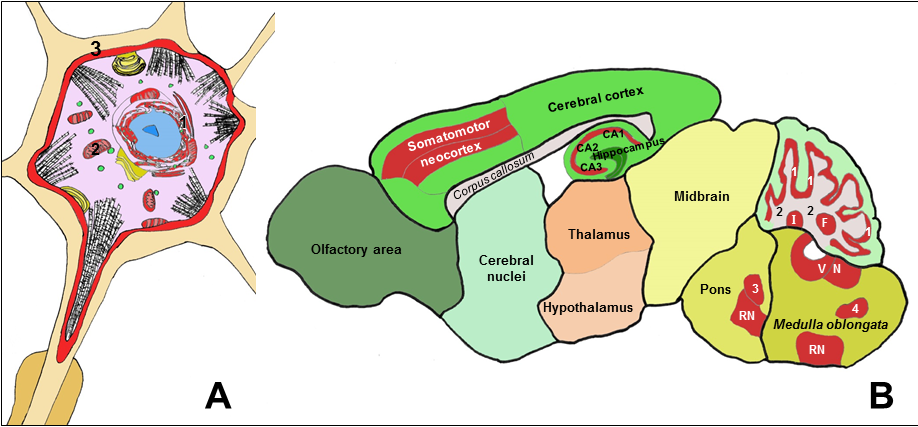
Figure 1: Schematic drawings showing a neuron (A) and a sagittal section of a rat brain (B), with neuronal structures and areas with high expression of Cb5R3 highlighted in red color.
A: Biochemical functions of the Cb5R/Cb5 redox system at cellular level in neurons:
(1) Endoplasmic reticulum: Fatty acids desaturation - Steroids and xenobiotics metabolism - Sialic acid metabolism - Modulation of methionine synthase - Protection against endoplasmic reticulum stress. (2) Mitochondria: Cholesterol and steroids metabolism - Biosynthesis of adenosylcobalamin - Electron shuttle with cytochrome c. (3) Plasma membrane: Extracellular ascorbate recycling - Antioxidant protection - Redox signaling in lipid rafts
B: Brain structures with higher levels of Cb5R3 expression to be likely affected by Cb5R functional deficiency or dysregulation:
Somatomotor neocortex: pyramidal neuronal tracks. Hippocampus: pyramidal CA1, CA2 and CA3 neurons. Cerebellum cortex: Purkinje and cerebellar granule neurons (1) - Efferent projections of Purkinje neurons (2). Cerebellum nuclei: Fastigial (F) – Interposed (I) – Dentate. Brain stem nuclei: pyramidal and gigantocellular neurons of vestibular nuclei (VN), reticular nuclei (RN), and motor nuclei: motor trigeminal nucleus (3), hypoglossal nucleus, dorsal motor nucleus of the vagus nerve, nucleus spinal tract of oral trigeminal nerve (4), ventral cochlear nucleus and root of facial nerve.
Finally, the Human Protein Atlas (www.proteinatlas.org/humanproteome/brain) and the Expression Atlas Genes of the EMBL-European Bioinformatics Institute (www.ebi.ac.uk/gxa/home) lists other Cb5R isoforms, i.e. Cb5R1, Cb5R2 and Cb5R4, as expressed proteins and genes in the human brain, respectively. However, experimental data are lacking to raise conclusions on the putative differential expression of these Cb5R isoforms in brain cells and neuronal structures.
This work has been supported by Grant BFU2014- 53641-P of the Spanish Plan Nacional de I+D+I and by Grants GR15139 and GR15127 of the Junta de Extremadura to Research Groups BBB008 and CTS005, both with co-financing by the European Funds for Structural Development (FEDER). Alejandro K. Samhan-Arias is supported by a Post-doctoral Fellowship SFRH/BPD/100069/2014 of the Fundação para a Ciência e Tecnologia, Portugal.