Relevance of Catalytic Autoantibodies to Myelin Basic Protein (MBP) in Autoimmune Disorders
Mario Gonzalez-Gronow1, 2*, Salvatore V. Pizzo2
1Department of Biological Sciences, Laboratory of Environmental Neurotoxicology, Faculty of Medicine, Universidad Católica del Norte, Coquimbo, Chile
2Department of Pathology, Duke University Medical Center, Durham, NC, USA
Abstract
Catalytic autoantibodies with proteinase enzymatic activity against myelin basic protein (MBP) are a distinctive feature of several autoimmune disorders. These autoantibodies, named abzymes (Abz), have both antibody and proteinase activity in a single molecule. Abz targeting MBP (MBP Abz) are commonly found in sera from multiple sclerosis (MS) and systemic lupus erythematosus (SLE) patients, and only recently have been identified in sera from autism spectrum disorder (ASD) patients. Their activities and specificity are similar in MS and SLE; however, although they recognize the same substrate, MBP, the catalytic activity of the Abz from autism spectrum disorder patients is controlled by different proteinase inhibitors. MBP Abz are generated as part of a process started by loss of compaction of myelin due to changes in charge after deamination of arginyl residues in MBP by the enzyme peptidylarginine deiminase. This exposes a normally hidden surface of MBP to T-cells initiating the autoimmune response. A large body of evidence suggests that MBP Abz play an important role in the pathogenesis not only of MS and SLE, but also of ASD. Many autoantibodies found in MS and SLE are also observed in healthy individuals at ranges usually considered pathological; however, clinical signs of the disease are not manifested, suggesting that expression of single autoantibodies may be inconsequential to develop the disease. However, it is the expression of hundreds of different autoantibodies, in addition to MBP Abz, that collectively lead to the clinical development of MS and SLE.
Introduction
Antigen-specific catalytic autoantibodies have been identified in several autoimmune diseases and their repertoire includes antibodies whose antigen binding sites may recognize external as well as autologous antigens and may structurally resemble the active site of enzymes displaying enzymatic activity1. Under normal physiological conditions, B cell clones that produce antibodies with catalytic activity are negatively regulated; however, their expression is enhanced following active immunization, or if the regulatory mechanisms controlling the catalytic antibody-producing B cell clones are perturbed, as is the case during the course of autoimmune diseases2. Catalytic autoantibodies or Abz possess both antibody and enzymatic activity in a single molecule and they are an important pathogenic factor in the progression of clinical autoimmune disorders3. One specific antigen, MBP, a component of the myelin sheath of neurons in the peripheral nervous system and central nervous system (CNS)4, is a target of Abz in several autoimmune disorders including MS5, SLE6, 7 and ASD8.
The degradation of MBP is a central feature of MS9. MBP, is usually compacted within the myelin sheath; however, in patients with immune disorders, MBP becomes accessible to catalysis by peptidyl arginine deiminase, which converts arginine residues into citrulline, decreasing both its charge and compact structure10. The loss of compaction facilitates access to the protein and proteolytic generation of immunodominant MBP peptides which sensitize T-cells for the autoimmune response to MBP11,12. Autoantibodies against MBP are also found in sera from patients with Alzheimer’s disease (AD)13 and patients with advanced stages of rheumatoid arthritis (RA)14; however, MBP Abz catalytic activity in sera from AD and RA patients has not been reported. Therefore, we will focus this mini review mainly on the pathogenic and clinical relevance of MBP Abz in MS, SLE and ASD.
Catalytic Autoantibodies to MBP in Multiple Sclerosis
MS is a chronic disease of the CNS characterized by loss of motor and sensory function, that result from immune-mediated inflammation, demyelination and subsequent axonal damage15. It is widely accepted that the inflammatory process in MS is caused by an autoimmune cascade, involving T-cells which target myelin self-antigens16, via a mechanism possibly involving molecular mimicry between cross-reactive antigens expressed by viruses and myelin components17. MBP Abz of the IgA, IgG and IgM classes are found in sera from MS patients18,19 and their catalytic activities correlate with the levels of disability observed in these patients20. The MS Abz cleave MBP at six preferential sites16, all of them located at the immediately COOH-terminal sides from basic amino acids, arginine and lysine, and positioned in the immunodominant MBP region Gln81-Gly10321. The mechanism of the MS MBP Abz catalysis is similar to that of serine proteinases; however, its activity is not inhibited by the serine proteinase inhibitor aprotinin, suggesting that this lack of inhibition makes the antibody-mediated catalysis an important factor in the neurodegeneration observed in MS22. For these reason, MBP Abz found in MS are biomarkers of disease progression.
Catalytic Autoantibodies to MBP in Systemic Lupus Erythematosus
SLE is a systemic autoimmune disease characterized by disorganization of conjunctive tissues resulting from damage to skin and visceral capillaries and the presence of a wide variety of autoantibodies23. The clinical and serological heterogeneity of SLE makes it difficult to diagnose at the very early stage, when an inadequate number of symptoms are present24. The most widely used classification criteria for SLE are those proposed by the American College of Rheumatology, published in 1971 and revised in 199725 (for an updated review see ref. 23).
SLE and MS share many of the clinical, biochemical and immunological features including the presence of MBP Abz. These SLE MBP Abz, mainly of the IgG class, show a high specificity for MBP alone6, and hydrolyze this protein at the same four immunodominant sites recognized by MS MBP Abz7. The immune systems of SLE patients can generate a variety of MBP Abz with different catalytic properties, which can attack MBP of myelin-proteolipid shell of axons7. MBP Abz are part of the intrinsic immune response observed in SLE26, and they play an important role in the diagnostic index of MS20; however, their pathogenic role in SLE remains inconclusive7. In addition to MBP Abz, SLE is associated with more than 100 different autoantibodies27. Many of these autoantibodies have been found in asymptomatic individuals28, suggesting that their presence is not pathological per se, and raises the possibility that autoreactivity is an important feature of a normal immune response29. The presence of autoreactivity in the B-cell population of healthy individuals is normally present and can be stimulated in vitro to produce pathogenic autoantibodies such as those found in SLE, suggesting that expression of autoantibodies by autoreactive B cells is an early step towards disease development30. Several factors that trigger expression of autoantibodies have been suggested including oestrogens, pesticides, bacterial superantigens, and viral31. In this context, the immune response to the MBP peptide Gln81-Gly103 is major target of both T and cell responses to MBP, a region with structural similarities in sequences found in peptides of several viruses including human papillomavirus7,13,40, cytomegalovirus, herpes simplex, Epstein-Barr virus, influenza A, hepatitis A and human adenovirus32. We constructed a model (Fig. 1) showing a hypothetical exposure of autoreactive B cells to agents containing antigens with structural homology to the MBP peptide Gln81-Gly103. This interaction stimulates expression of specific MBP Abz which move across the blood-brain barrier (BBB) and infiltrate the protective myelin sheath of brain neurons, leading to tissue injury. As observed in MS33, such mechanism may also function in SLE.
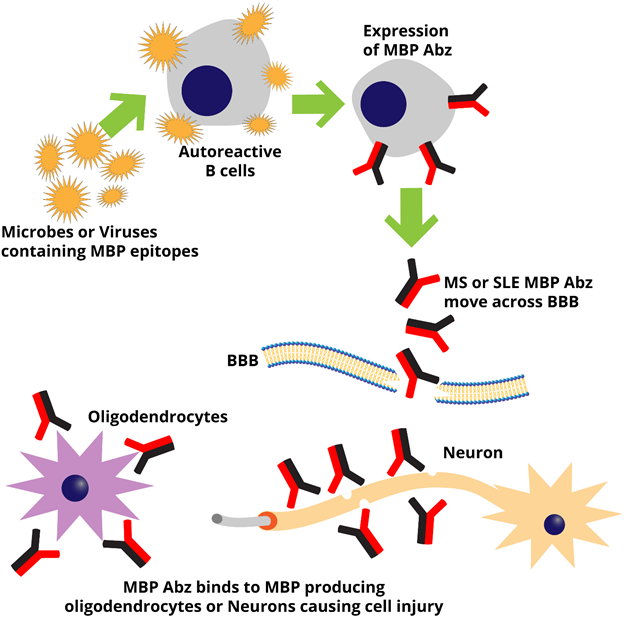
Figure 1: Expression and access of MBP Abz to brain tissue in MS and SLE. Microbes or viruses containing proteins with MBP epitopes interact with autoreactive B cells inducing expression of MBP Abz. These autoantibodies move across the blood-brain barrier (BBB) and bind to MBP exposed on the surface of neurons or MBP-expressing oligodendrocytes leading to cell injury.
Catalytic Autoantibodies to MBP in Autism Spectrum Disorders
ASD are a heterogeneous group of neurodevelopmental conditions characterized by unusual repetitive behaviors and impaired social and communication skills34. Although the etiology of ASD is complex, increasing evidence suggests that immune dysfunctions and the presence of autoantibodies to brain proteins are associated with behaviors observed in ASD35, as reflected by the high correlation between the titers of serum antibodies against brain proteins and the severity of the ASD36. Autoantibodies from individuals with ASD react with a wide variety of proteins in different regions of the brain37, and one of these autoantibodies targets MBP38. ASD patients show IgA and IgG class autoantibodies to MBP, but not of the IgM class8. An evaluation of their specificity revealed that the IgA autoantibodies against MBP exhibit the catalytic activity of a serine proteinase and are able to hydrolyze MBP in vitro8. Unlike the MBP Abz found in MS, the ASD MBP Abz activity is inhibited by the serine proteinase inhibitor aprotinin8. The ASD MBP Abz of the IgA class induces a decrease in long-term potentiation (LTP) in models of brain synaptic plasticity in isolated rat hippocampal slices perfused in vitro with the antibody or in an in vivo model of hippocampal slices from rats injected with the ASD IgA for 10 days8. A hypothetical model (Fig. 2) depicts how ASD MBP Abz move across the BBB and reach the brain causing synaptic dysfunctions which cause the decrease in synaptic plasticity (lower LTP) observed in the rat animal model8. Table 1 shows a summary of the biochemical properties of MBP Abz in MS, SLE and ASD.
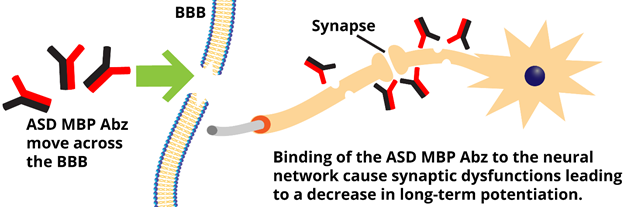
Figure 2: Access of MBP Abz to brain tissue in ASD. MBP Abz produced in the central nervous system move across the blood-brain barrier (BBB), reach brain tissues and bind to neurons. This interaction causes synaptic dysfunctions that affect synaptic plasticity inducing a decrease in long-term potentiation (LTP).
Table 1: Biochemical properties of catalytic autoantibodies to MBP in autoimmune diseases
| Function | MS | SLE | ASD |
| Autoantibodies of the IgA, IgG and IgM class | +++ 18, 19 | +++ 6 | +++ 8 |
| Recognition of the MBP region N81-G103 | +++ 21 | +++ 7 | N.D. |
| Cleavage of MBP | +++ 16, 21 | +++ 7 | +++ 8 |
| Correlation of titers with disability levels | +++ 20 | N.D. | N.D. |
N.D. non-determined
Conclusions
MBP Abz able to hydrolyze MBP have been found in MS, SLE and ASD. These Abz have been extensively studied in both MS and SLE; however, their presence in ASD have been identified only recently. The experimental data from MS and SLE permits the inclusion of them as important players in the pathogenesis of these two disorders. Many autoantibodies found in MS and SLE are also observed in healthy individuals at ranges usually considered pathological; however, their toxicity was not manifested, suggesting that expression of single autoantibodies may be inconsequential to develop the disease. However, there are hundreds of antigens affected by the immune response in these diseases, which in addition to MBP Abz, may collectively affect the clinical development of MS and SLE. Future studies should help to strengthen the understanding of the mechanisms involving MBP Abz in the pathogenesis of MS, SLE and ASD and improve the efficacy of new therapeutic strategies.
Acknowledgement
This work was supported by a grant from Fondo Nacional de Desarrollo Científico y Tecnológico de Chile, FONDECYT No 1130451.
References
- Tramontano A, Gololobob G, Paul G. Proteolytic antibodies: origins, selection and induction. In: Paul S (ed) Chemical Immunology: catalytic antibodies b Karger Basel. 2000; Vol 77: 1-17.
- Lacroix-Desmazes S, Wootla B, Delignat S, et al. Pathophysiology of catalytic antibodies. Immunol Lett. 2006; 103(1): 3-7.
- Gabibov AG, Ponomarenko NA, Tretyak EB, et al. Catalytic autoantibodies in clinical autoimmunity and modern medicine. Autoimmun Rev. 2006; 5(5): 324-330.
- Aggarwal S, Yurlova L, Simons M. Central nervous system myelin: structure, synthesis and assembly. Trends Cell Biol. 2011; 21(10): 585-93.
- Ponomarenko NA, Durova OM, Voroblev, et al. Autoantibodies to myelin basic protein catalyze site-specific degradation of their antigen. Proc Natl Acad Sci USA. 2006; 103(2): 281-286.
- Bezuglova A, Konenkova LP, Doronin BM, et al. Affinity and catalytic heterogeneity and metal-dependence of polyclonal myelin basic protein-hydrolyzing IgGs from sera of patients with systemic lupus erythematosus. J Mol Recognit. 2011; 24(6): 960-974.
- Timofeeva AM, Dmitrenok PS, Konenkova LP, et al. Multiple sites of the cleavage of 21- and 25-mer encephalytogenic oligopeptides corresponding to human myelin basic protein (MBP) by specific anti-MBP antibodies from patients with systemic lupus erythematosus. PLOS one. 2013; 8(3): e51600, 1-13.
- Gonzalez-Gronow M, Cuchacovich M, Francos, et al. Catalytic autoantibodies against myelin basic protein (MBP) isolated from serum of autistic children impair in vitro models of synaptic plasticity in rat hippocampus. J Neuroimmunol. 2015; 287(1): 1-8.
- Hafler DA, Slavik JM, Anderson DE, et al. Multiple sclerosis. Immunol Rev. 2005; 204(1): 208-231.
- Harauz G, Ishiyama N, Hill CM, et al.. Myelin basic protein-diverse conformational states of an intrinsically unstructured protein and its roles in myelin assembly and multiple sclerosis. Micron. 2004; 35(7): 503-42.
- Pritzker LB, Joshi S, Gowan JJ, et al. Deimination of myelin basic protein. 1. Effect of deimination of arginyl residues of myelin basic protein on its structure and susceptibility to digestion by cathepsin D. Biochemistry. 2000; 39(18): 5374-8.
- Musse AA, Boggs JM, Harauz G. Deimination of membrane-bound myelin basic protein in multiple sclerosis exposes an immunodominant epitope. Proc Natl Acad Sci U S A. 2006; 103(12): 4422-4427.
- Singh, VK, Yang, YY, Singh, EA (1992) Immunoblot detection of antibodies to myelin basic protein in Alzheimer’s disease patients. Neurosci Lett. 1992; 147(1): 25-28.
- Almeida Baptista TS, Esteves Petersen L, Molina JK, et al. Autoantibodies against myelin sheath and S100β are associated with cognitive dysfunction in patients with rheumatoid arthritis. Clin Rheumatol. 2017; 36(9): 1959-1968.
- Karussis D. The diagnosis of multiple sclerosis and the various related demyelinating syndromes: A critical review. J Autoimmun. 2014; 48-49(1): 134-142.
- Allegretta M, Nicklas JA, Sriram S, et al. T cells responsive to myelin basic protein in patients with multiple sclerosis. Science. 1990; 247(4943): 718-721.
- Wucherpfenning KW, Strominger JL. Molecular mimicry in T-cell-mediated autoimmunity: viral peptides activate human T cell clones specific for myelin basic protein. Cell. 1995; 80(5): 695-705.
- Polosukhina DI, Kanyshkova T, Doronin BM, et al. Hydrolysis of myelin basic protein by polyclonal catalytic IgGs from the sera of patients with multiple sclerosis. J Cell Mol Med. 2004; 8(3): 359-68.
- Polosukhina DI, Buneva VN, Doronin BM, et al. Hydrolysis of myelin basic protein by IgM and IgA antibodies from the sera of patients with multiple sclerosis. Med Sci Monit. 2005; 11(8): BR266-72.
- Ponomarenko NA, Durova OM, Vorobiev II, et al. Catalytic activity of autoantibodies toward myelin basic protein correlates with the scores on the multiple sclerosis expanded disability status scale. Immunol Lett. 2006; 103(1): 45-50.
- Belogurov A Jr, Kozyr A, Ponomarenko N, et al. Catalytic antibodies: balancing between Dr. Jekyll and Mr. Hyde. Bioessays. 31(11): 1161-71.
- Belogurov AA Jr, Kurkova IN, Friboulet A, et al. Recognition and degradation of myelin basic protein peptides by serum autoantibodies: novel biomarker for multiple sclerosis. J Immunol. 2008; 180(2): 1258-1267.
- Yu C, Gershwin ME, Chang C. Diagnostic criteria for systemic lupus erythematosus: a critical review. J Autoimmun. 2014; 48-49(1): 10-13.
- Aringer M, Günther C, Lee-Kirsch MA. Innate immune processes in lupus erythematosus. Clin Immunol. 2013; 147(3): 216-22.
- Hochberg MC. Updating the American College of Rheumatyology criteria for the classification of systemic lupus erythematosus. Arthritis Rheum. 1997; 40(9): 1725.
- Bezuglova AM, Konenkova LP, Buneva VN, et al. IgGs containing light chains of the λ- and κ- type and of all subclasses (IgG1-IgG4) from the sera of patients with systemic lupus erythematosus hydrolyze myelin basic protein. Int Immunol. 2012; 24(12): 759-770.
- Sherer Y, Gorstein A, Fritzler MJ, et al. Autoantibody explosion in systemic lupus erythematosus: more than 100 different antibodies found in SLE patients. Semin Arthritis Rheum. 2004; 34(2): 501-37.
- Li QZ, Karp DR, Quan J, et al. Risk factors for ANA positivity in healthy persons. Arthritis Res Ther. 2011; 13(2): R38.
- Olsen NJ, Karp DR. Autoantibodies and SLE: the threshold for disease. Nat Rev Rheumatol. 2014; 10(3): 181-6.
- Aringer M, Vital E. Lots of autoantibodies equal lupus. Arthritis Res Ther. 2013; 15(1): 102.
- Olsen NJ, Karp DR. Autoantibodies and SLE: the threshold for disease. Nat Rev Rheumatol. 2014; 10(3): 181-6.
- Steinman L. Antigen-specific therapy of multiple sclerosis: the long-sought magic bullet. Neurotherapeutics. 4(4): 661-5.
- O'Connor KC, Bar-Or A, Hafler DA. The neuroimmunology of multiple sclerosis: possible roles of T and B lymphocytes in immunopathogenesis. J Clin Immunol. 2001; 21(2): 81-92.
- Volkmar FR, Pauls D. Autism. Lancet. 2003; 362(9390): 1133-1141.
- Careaga M, Ashwood P. Autism spectrum disorders: from immunity to behavior. Methods Mol Biol. 2012; 934(1): 219-40.
- Edmiston E, Ashwood P, Van de Water J. Autoimmunity, Autoantibodies, and Autism Spectrum Disorder. Biol Psychiatry. 2017; 81(5): 383-390.
- Mora M, Quintero L, Cardenas R, et al. Association between HSV-2 infection and serum anti-rat brain antibodies in patients with autism. Invest Clin. 2009; 50(3): 315-26.
- Singh VK, Warren RP, Odell JD, et al. Antibodies to myelin basic protein in children with autistic behavior. Brain Behav Immun. 1993; 7(1): 97-103.
